ALLMPUS PROVIDE PRODUCT ERYTHROMYCIN IMPURITY WITH ALL RELATED DOCUMENTS INCLUDING MSDS/SDS, SER REPORT, COA, HPLC, MASS, 13CNMR, 1HNMR AND TGA POTENCY (TGA UPTO 800 DEG C) AND ARE ACCEPTABLE TO ALL REGULATORY AGENCIES & ALSO USED FOR ANDA FILING /DMF FILING AND GENOTOXIC STUDY.
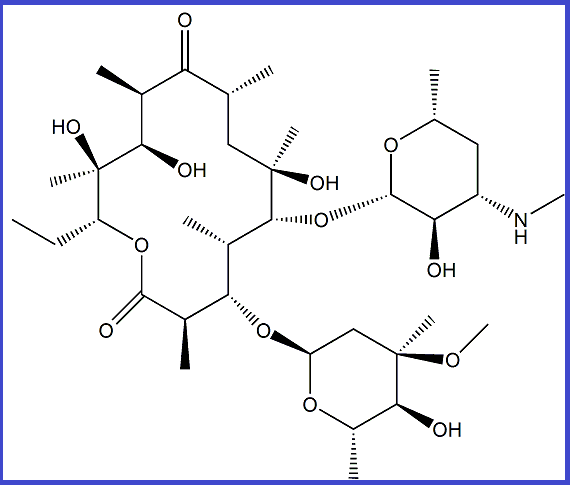
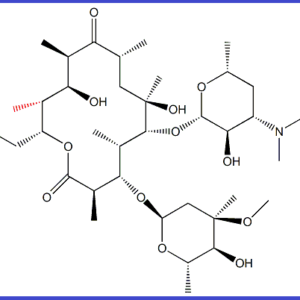
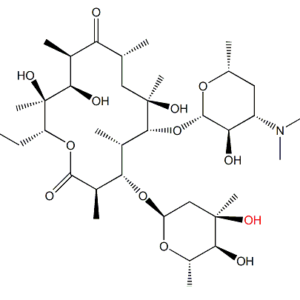
DESCRIPTION – Erythromycin Ep Impurity B is manufactured by ALLMPUS LABORATRIES PVT. Ltd. And are delivered and packaged according to regulations and Non-Infectious Biology Material and is purely for research purpose only not for human consumption. All documents as per pharmacopeia specification including MSDS/SDS, SER REPORT, COA, HPLC, MASS, 13CNMR, 1HNMR and TGA potency (Upto 800 deg c) and are acceptable to all various regulatory authorities such as ICH, USFDA, Canadian Drug and Health Agency & also used for ANDA filing /DMF filing and genotoxic study The materials are used purely for Testing, R & D and analytical Research Purposes and the Material is not from livestock or avian species and has not been exposed to livestock or avian disease agents. The Material is not immunogen and does not come from a facility where work with any viruses is done. The Material is not recombinant and do not contain any genes associated with livestock or poultry (or livestock or poultry diseases). If there is any problem, issue or question about this product, please contact info@allmpus.com –
Application – ERYTHROMYCIN EP IMPURITY B / ERYTHROMYCIN USP RC N, ERYTHROMYCIN IMPURITY STANDARD, ERYTHROMYCIN IMPURITY SUPPLIER, ERYTHROMYCIN PHARMACOPIA IMPURITY STANDARD, PHARMACEUTICAL IMPURITY SUPPLIER



Avaliações
Não há avaliações ainda.